Teknologi material membran memainkan peran penting dalam perlindungan lingkungan, energi, biomedis, dan bidang lainnya. Polivinil alkohol (PVA) PVA telah menjadi target utama penelitian material membran karena kelarutannya yang sangat baik dalam air, sifat pembentuk film, dan biokompatibilitasnya. Namun, karena konsentrasi gugus hidroksil yang tinggi dalam rantai molekulnya, PVA mudah membengkak atau larut dalam lingkungan dengan kelembapan tinggi, sehingga memengaruhi stabilitasnya dalam aplikasi yang kompleks. Untuk mengatasi keterbatasan ini, penelitian tentang Polivinil Alkohol yang Dimodifikasi telah meningkat dalam beberapa tahun terakhir. Melalui ikatan silang kimia, pencampuran, dan penambahan pengisi anorganik, ketahanan air, sifat mekanik, dan stabilitas kimia Film polivinil alkohol (film PVA) Telah ditingkatkan secara signifikan. Membran PVA yang dimodifikasi telah menemukan aplikasi yang luas dalam pengolahan air, sel bahan bakar, pemisahan gas, dan bidang lainnya. Meningkatnya teknologi modifikasi yang ramah lingkungan dan ramah lingkungan telah memberikan membran PVA potensi yang lebih besar untuk aplikasi yang ramah lingkungan dan terurai secara hayati. Dengan mengoptimalkan proses produksi dan memperluas strategi modifikasi fungsional, membran PVA akan memainkan peran yang lebih signifikan dalam bidang material membran berkinerja tinggi.
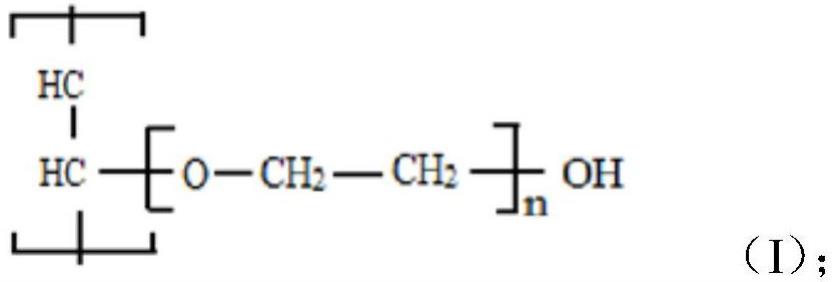
1. Metode Modifikasi Polivinil Alkohol
1.1 Ikatan Silang Kimia
Polivinil alkohol (PVA) adalah polimer yang sangat polar. Karena banyaknya gugus hidroksil pada rangka dasarnya, ia mudah membentuk ikatan hidrogen dengan molekul air, menyebabkannya membengkak atau bahkan larut dalam lingkungan lembap. Hal ini secara signifikan membatasi stabilitasnya dalam aplikasi tertentu. Ikatan silang kimia merupakan metode yang efektif. Dengan memasukkan ikatan silang antar rantai molekul PVA, terbentuklah jaringan tiga dimensi yang stabil, sehingga mengurangi kelarutannya dalam air dan meningkatkan ketahanan air serta stabilitas termalnya. Ikatan silang biasanya melibatkan pembentukan ikatan kovalen antar molekul PVA, sehingga rantai polimer kurang terdispersi dalam air. Agen pengikat silang yang umum meliputi aldehida (seperti glutaraldehida), epoksida (seperti epiklorohidrin), dan poliasam (seperti asam sitrat dan maleat anhidrida). Agen pengikat silang yang berbeda memengaruhi pola ikatan silang dan sifat polimer yang dimodifikasi. Misalnya, ketika glutaraldehida bertemu dengan gugus hidroksil PVA dalam lingkungan asam, mereka membentuk struktur ikatan silang yang solid. Selain itu, maleat anhidrida dapat mengikat bagian-bagian PVA melalui esterifikasi, yang sangat membantu PVA menahan air. Karena film PVA yang terikat silang ini memiliki ikatan antar molekul yang lebih kuat, film ini dapat menahan panas lebih banyak, terbukti dari suhu transisi gelas (Tg) dan suhu dekomposisi termal (Td) yang lebih tinggi.
1.2 Modifikasi Pencampuran
Modifikasi pencampuran merupakan metode penting lainnya untuk meningkatkan kinerja film PVA. Dengan pencampuran dengan polimer lain, sifat mekanik, ketahanan air, dan stabilitas kimia PVA dapat dioptimalkan. Karena sifat hidrofilik PVA, pencampuran langsung dengan polimer hidrofobik dapat menimbulkan masalah kompatibilitas. Oleh karena itu, penting untuk memilih bahan pencampuran yang tepat dan mengoptimalkan proses pencampuran. Misalnya, ketika dicampur dengan polivinil butiral (PVB), sifat hidrofobisitas PVB memungkinkan film PVA mempertahankan stabilitas morfologi yang baik bahkan di lingkungan dengan kelembapan tinggi. Lebih lanjut, suhu transisi gelas PVB yang tinggi meningkatkan ketahanan panas film hasil pencampuran. Pencampuran dengan polivinilidena fluorida (PVDF) secara signifikan meningkatkan sifat hidrofobisitas film PVA. Lebih lanjut, ketahanan kimia PVDF yang sangat baik memungkinkan film hasil pencampuran tetap stabil bahkan di lingkungan kimia yang kompleks. PVA juga dapat dicampur dengan polietersulfon (PES) dan poliakrilonitril (PAN) untuk meningkatkan permeabilitas selektif membran, membuatnya lebih dapat diaplikasikan secara luas dalam membran pemisahan gas dan pemurnian air.
2. Aplikasi Membran Modifikasi PVA pada Material Membran Berkinerja Tinggi
2.1 Membran Pengolahan Air
Pengembangan teknologi membran pengolahan air sangat penting untuk mengatasi kekurangan sumber daya air dan meningkatkan kualitas serta keamanan air. Membran PVA bekerja sangat baik sebagai film dan dapat menyatu dengan jaringan hidup, sehingga dapat digunakan dalam berbagai macam pemisahan membran seperti ultrafiltrasi, nanofiltrasi, dan osmosis terbalik. Namun, karena PVA menyukai air dan larut di dalamnya, ia dapat rusak seiring waktu. Hal ini membuat membran lebih lemah dan tidak tahan lama. Itulah sebabnya modifikasi membran PVA menjadi fokus utama dalam penelitian pengolahan air. Ikatan silang kimia merupakan teknologi kunci untuk meningkatkan ketahanan air membran PVA. Agen ikatan silang (seperti glutaraldehida dan maleat anhidrida) membentuk ikatan kimia yang stabil antar rantai molekul PVA, menjaga morfologi membran tetap stabil di lingkungan berair dan memperpanjang masa pakainya. Selain itu, penambahan pengisi anorganik juga merupakan cara penting untuk meningkatkan ketahanan hidrolisis dan kekuatan mekanis membran PVA. Penambahan nano-silika (SiO₂) dan nano-alumina (Al₂O₃) dapat menciptakan campuran yang kuat pada material membran. Hal ini membuat membran lebih tahan terhadap kerusakan akibat air dan meningkatkan kekuatannya. Dengan demikian, membran tetap berfungsi dengan baik bahkan pada tekanan tinggi. Selain itu, pencampuran PVA dengan polimer lain seperti polietersulfon (PES) dan polivinilidena fluorida (PVDF) membuat membran lebih tahan air dan lebih tahan terhadap pengotoran. Ini berarti membran lebih awet dan laju alirannya tetap terjaga, bahkan dengan penumpukan kotoran.
2.2 Membran Pertukaran Proton untuk Sel Bahan Bakar
Sel bahan bakar adalah perangkat konversi energi yang bersih dan efisien, dan membran pertukaran proton, sebagai komponen intinya, menentukan kinerja dan masa pakainya. PVA, karena sifat pembentuk film dan kemampuan prosesnya yang sangat baik, merupakan kandidat yang menjanjikan untuk membran pertukaran proton. Namun, konduktivitas protonnya yang rendah dalam keadaan mentah menyulitkan pemenuhan persyaratan efisiensi tinggi sel bahan bakar, sehingga memerlukan modifikasi untuk meningkatkan konduktivitas proton. Modifikasi sulfonasi merupakan salah satu metode kunci untuk meningkatkan konduktivitas proton membran PVA. Untuk meningkatkan kemampuan membran menyerap air dan membantu proton bergerak lebih baik, kami menambahkan asam sulfonat ke rantai PVA. Hal ini menciptakan saluran air yang kontinu. Mencampurnya juga dapat membantu. Jika Anda mencampur PVA dengan SPS dan SPEEK, keduanya membentuk jaringan yang membantu pertukaran proton dan membuat membran lebih kuat. Namun, penggunaan membran PVA dalam DMFC memiliki masalah tersendiri. Metanol dapat bocor, membuang-buang bahan bakar dan memperburuk keadaan. Untuk mengatasi hal ini, para ilmuwan telah menambahkan bahan-bahan seperti silika tersulfonasi dan nanopartikel zirkonia ke membran PVA. Mereka juga menggunakan lapisan untuk menghalangi metanol melewati membran dan mengurangi kebocoran.
3. Tren dan Tantangan Pembangunan
3.1 Pengembangan Teknologi Modifikasi Hijau dan Ramah Lingkungan
Dengan semakin ketatnya peraturan lingkungan dan semakin banyaknya penerapan konsep pembangunan berkelanjutan, teknologi modifikasi film PVA yang ramah lingkungan dan ramah lingkungan telah menjadi fokus penelitian utama. Penelitian tentang film PVA yang dapat terurai secara hayati telah mencapai kemajuan yang signifikan dalam beberapa tahun terakhir. Dengan pencampuran dengan polimer alami (seperti kitosan, pati, dan selulosa) atau pengenalan nanofiller yang dapat terurai secara hayati (seperti hidroksiapatit dan nanoselulosa berbasis bio), biodegradabilitas film PVA dapat ditingkatkan secara signifikan, membuatnya lebih mudah terurai di lingkungan alami dan mengurangi polusi pada ekosistem. Lebih lanjut, untuk mengurangi dampak lingkungan dan manusia dari bahan kimia beracun yang digunakan dalam proses modifikasi ikatan silang tradisional, para peneliti telah mulai mengembangkan agen ikatan silang yang tidak beracun dan proses modifikasi yang lebih ramah lingkungan. Ini termasuk ikatan silang kimia menggunakan pengikat silang alami seperti asam sitrat dan kitosan, dan metode modifikasi fisik seperti sinar ultraviolet dan perawatan plasma, mencapai ikatan silang bebas polusi. Teknologi modifikasi hijau ini tidak hanya meningkatkan keramahan lingkungan dari film PVA tetapi juga meningkatkan nilai aplikasinya dalam pengemasan makanan, biomedis, dan bidang lainnya, menjadikannya arah utama untuk pengembangan bahan membran polimer di masa depan.
3.2 Tantangan dan Solusi untuk Aplikasi Industri
Meskipun film PVA yang dimodifikasi memiliki prospek aplikasi yang luas di bidang material membran berkinerja tinggi, film ini masih menghadapi berbagai tantangan dalam industrialisasinya. Biaya produksi yang tinggi menjadi hambatan utama, terutama untuk film PVA yang menggunakan nanofiller atau modifikasi khusus. Bahan baku yang mahal dan proses preparasi yang kompleks membatasi produksi skala besar. Optimalisasi proses masih memerlukan perbaikan. Saat ini, beberapa metode modifikasi memiliki konsumsi energi yang tinggi dan siklus produksi yang panjang, sehingga menghambat kelayakan ekonomi dan kelayakan produksi industri. Untuk mengatasi masalah ini, upaya ke depan akan difokuskan pada pengembangan proses preparasi yang efisien dan berbiaya rendah, seperti penerapan teknik sintesis air yang ramah lingkungan untuk meningkatkan efisiensi produksi, sekaligus mengoptimalkan sistem pencampuran untuk meningkatkan stabilitas kinerja film PVA. Lebih lanjut, arah pengembangan film PVA berkinerja tinggi ke depan akan berfokus pada peningkatan daya tahan, pengurangan konsumsi energi produksi, dan perluasan fungsionalitas cerdas. Misalnya, pengembangan film PVA cerdas yang dapat merespons stimulus eksternal (seperti perubahan suhu dan pH) untuk memenuhi berbagai kebutuhan industri dan biomedis.
4. Kesimpulan
Polivinil alkohol (PVA), sebagai polimer berkinerja tinggi, memiliki prospek aplikasi yang luas di bidang material membran. Film PVA dapat dibuat lebih kuat dan lebih tahan terhadap unsur-unsur alam dengan menggunakan metode seperti ikatan silang kimia, ko-modifikasi, dan penambahan pengisi anorganik. Hal ini membuatnya cocok untuk berbagai keperluan seperti pengolahan air dan sel bahan bakar. Selain itu, teknologi modifikasi ramah lingkungan yang baru telah membuat film PVA lebih mudah terurai dan kurang beracun. Ini berarti film PVA dapat berperan besar dalam perlindungan lingkungan dan penggunaan medis. Di masa mendatang, aplikasi industri masih akan menghadapi tantangan dalam hal biaya produksi dan optimalisasi proses. Peningkatan lebih lanjut dalam efisiensi ekonomi dan kelayakan teknologi modifikasi diperlukan untuk mendorong penerapan film PVA secara luas di bidang material membran berkinerja tinggi dan menyediakan solusi material membran berkualitas tinggi untuk pembangunan berkelanjutan.
Situs web: www.elephchem.com
WhatsApp: (+)86 13851435272
Surel: admin@elephchem.com